Uniquely Enabling Mechanism for Bis-oxazoline Copper(II)-Catalyzed Azidation of Pyranosides and Furanosides
Abstract
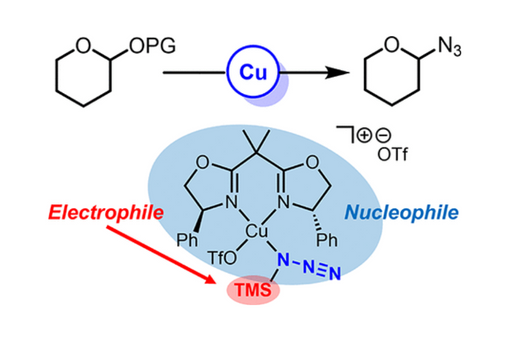
Azidoglycosides are important intermediates for the synthesis of amino sugars, glycosylamines, and N-glycoproteins. This work reports a bis-oxazoline Cu2+ catalyst for the selective azidation of pyranosides and furanosides. Mechanistic studies revealed that the Lewis acid catalyst does not coordinate and activate the sugar in the typical bidentate fashion but instead activates TMS azide as part of a silylium transfer (R3Si+) mechanism. A combination of UV–vis and in situ NMR studies pointed to monodentate TMS-azide coordination to copper followed by speciation to TMS-OTf (from a triflate counteranion) and a divalent (BOX)Cu-N3 nucleophile. Two pathways for catalytic turnover are proposed, namely, a more rapid cycle wherein the triflate acts as a silylium carrier (via TMS-OTf) to activate the sugar and a slower cycle where silylium transfer to the sugar occurs directly from the TMS-azide catalyst complex. In both cases, the azide nucleophile is a (BOX)Cu-N3 complex. The proposed mechanisms were also probed with computations to elucidate the structure of key putative intermediates.
Citation
Uniquely Enabling Mechanism for Bis-oxazoline Copper(II)-Catalyzed Azidation of Pyranosides and Furanosides
Neyen Romano, Nicholas M. Hein, Kevin Basemann, Youngran Seo, and Michel R. Gagné
ACS Catalysis 2022 12 (20), 12500-12510
DOI: 10.1021/acscatal.2c04208