Tuning the Three-Phase Microenvironment Geometry Promotes Phase Formation
Abstract
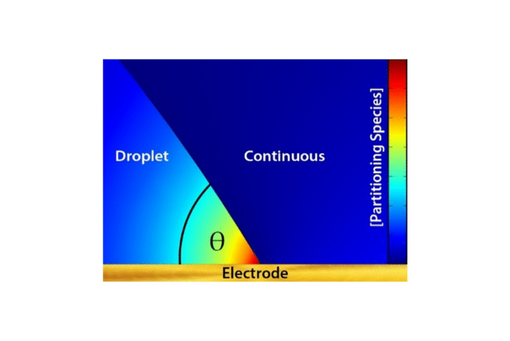
Nature builds multiphase environments to drive specific reactivity across boundaries. Multiphase systems present an opportunity to drive reactions that would otherwise not occur in bulk, continuous phases. Here, we demonstrate preferential nucleation near the three-phase boundary as a function of its geometry. A submicroliter water droplet deposited on an electrode immersed in a continuous 1,2-dichloroethane (DCE) phase is used to fabricate a three-phase junction (water|DCE|electrode). Adjusting the angle of the three-phase junction by changing the hydrophilicity of the electrode can lead to precipitation of ferrocenemethanol (FcMeOH) at the three-phase boundary only. Analysis by cyclic voltammetry coupled to numerical simulations provides insight into the physicochemical origin of the precipitation depending on the three-phase boundary angle. This finding offers a convenient means to control the local reactivity at three-phase boundaries.
Citation
Tuning the Three-Phase Microenvironment Geometry Promotes Phase Formation
Guillermo S. Colón-Quintana, Kathryn J. Vannoy, Christophe Renault, Silvia Voci, and Jeffrey E. Dick
The Journal of Physical Chemistry C 2022 126 (47), 20004-20010
DOI: 10.1021/acs.jpcc.2c03973