Precyclization Conformer Profiles of −SiR3+- and −Bcat+-Activated Linear Si-Protected Hexitols Explain Condensative Cyclization Selectivities
Abstract
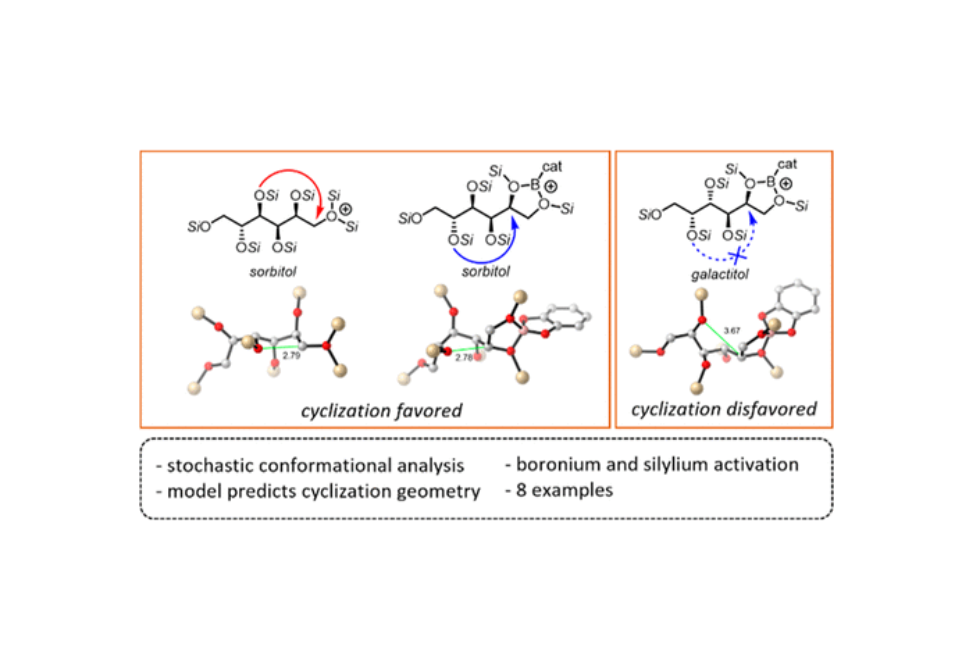
The condensative cyclization of sp3 C–O bonds in per-silylated hexitols is investigated by computation. Conformer searches using the Monte Carlo algorithm, followed by successively higher levels of theory (MMFF, PM3, and B3LYP), of −SiR3+- and −Bcat+-activated substrates lead to structures primed for intramolecular chemistry. Silane activation features O4 to C1 attack, while borane activation suggests boronium ions that activate O5 to C2 reactivity. This, in conjunction with Boltzmann population analysis, parallels reported reactivity for sorbitol, mannitol, and galactitol. Calculations using the meta-hybrid M06-2X functional additionally provide free-energy profiles for each cyclization event. In most of the cases presented, precyclization conformers that position a nucleophilic oxygen less than 3.0 Å from the C–O leaving group correlate to efficient experimental reactivities. Two examples of galactitol containing bridging silyl groups are analyzed computationally, and the experimental outcomes match predictions. The computational regime presented is a step closer to providing predictive power for the reduction of per-functionalized molecules.
Citation
Precyclization Conformer Profiles of −SiR3+- and −Bcat+-Activated Linear Si-Protected Hexitols Explain Condensative Cyclization Selectivities
Jared M. Lowe, Youngran Seo, Joshua J. Clarke, and Michel R. Gagné
The Journal of Organic Chemistry 2022 87 (18), 12065-12071
DOI: 10.1021/acs.joc.2c01151