Mechanisms of Electrochemical N2 Splitting by a Molybdenum Pincer Complex
Abstract
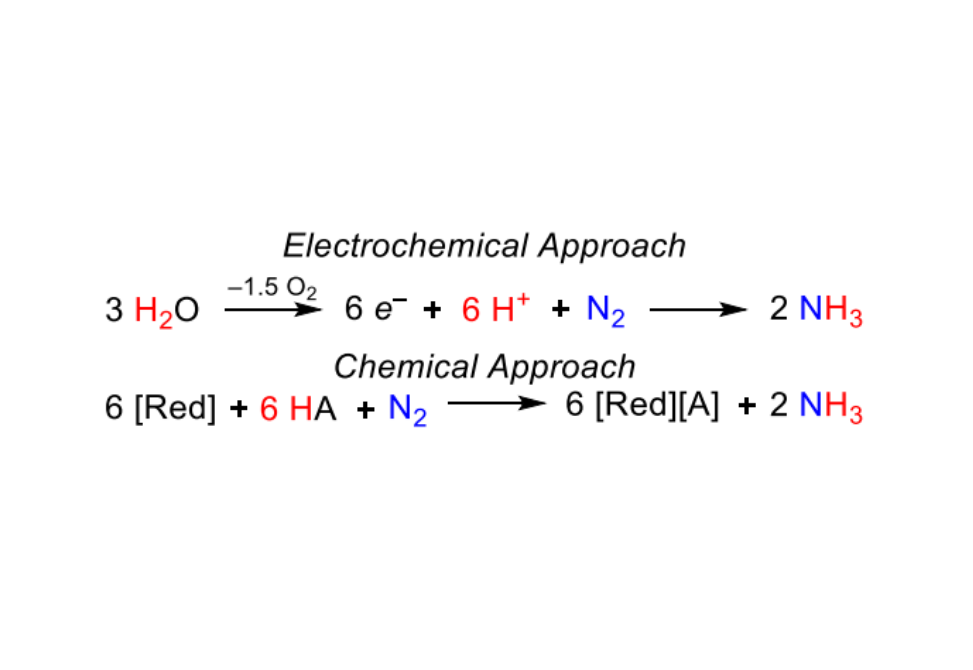
Molybdenum complexes supported by tridentate pincer ligands are exceptional catalysts for dinitrogen fixation using chemical reductants, but little is known about their prospects for electrochemical reduction of dinitrogen. The viability of electrochemical N2 binding and splitting by a molybdenum(III) pincer complex, (pyPNP)MoBr3 (pyPNP = 2,6-bis(tBu2PCH2)-C5H3N)), is established in this work, providing a foundation for a detailed mechanistic study of electrode-driven formation of the nitride complex (pyPNP)Mo(N)Br. Electrochemical kinetic analysis, optical and vibrational spectroelectrochemical monitoring, and computational studies point to two reaction pathways: in the “reaction layer” pathway, the molybdenum(III) precursor is reduced by 2e– and generates a bimetallic molybdenum(I) Mo2(-N2) species capable of N–N bond scission. In the “bulk solution” pathway the precursor is reduced by 3e– at the electrode surface to generate molybdenum(0) species that undergo chemical redox reactions via comproportionation in the bulk solution away from the electrode surface to generate the same bimetallic molybdenum(I) species capable of N2 cleavage. The comproportionation reactions reveal the surprising intermediacy of dimolybdenum(0) complex trans,trans-[(pyPNP)Mo(N2)2](-N2) in N2 splitting pathways. The same “over-reduced” molybdenum(0) species was also found to cleave N2 upon addition of lutidinium, an acid frequently used in catalytic reduction of dinitrogen.
Citation
Mechanisms of Electrochemical N2 Splitting by a Molybdenum Pincer Complex
Quinton J. Bruch, Santanu Malakar, Alan S. Goldman, Alexander J. M. Miller
Inorganic Chemistry, Volume 61, Issue 4
Published Jan 18, 2022