Enantio- and Diastereoselective Mannich Reactions of ß-Dicarbonyls by Second Stage Diastereoconvergent Crystallization
Abstract
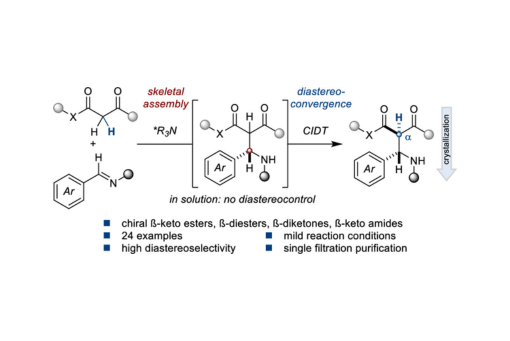
The synthesis of chiral α-monosubstituted-ß-dicarbonyls is a challenging task in asymmetric catalysis due to the rapid, typically uncontrolled, product racemization or epimerization under most reaction conditions. For this reason, diastereoselective additions of unsubstituted ß-dicarbonyls to π-electrophiles are unusual. Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution. Mechanistic studies suggest a scenario where the initial enantioselective, diastereodivergent skeletal assembly is catalyzed by a chiral tertiary amine organocatalyst, which then facilitates second stage crystallization-induced diastereoconvergence to provide the challenging α-stereocenter in excellent stereoselectivity.
Citation
Enantio- and Diastereoselective Mannich Reactions of ß-Dicarbonyls by Second Stage Diastereoconvergent Crystallization
William R. Cassels, Evan T. Crawford, and Jeffrey S. Johnson
ACS Catalysis 2023 13 (10), 6518-6524
DOI: 10.1021/acscatal.3c01515