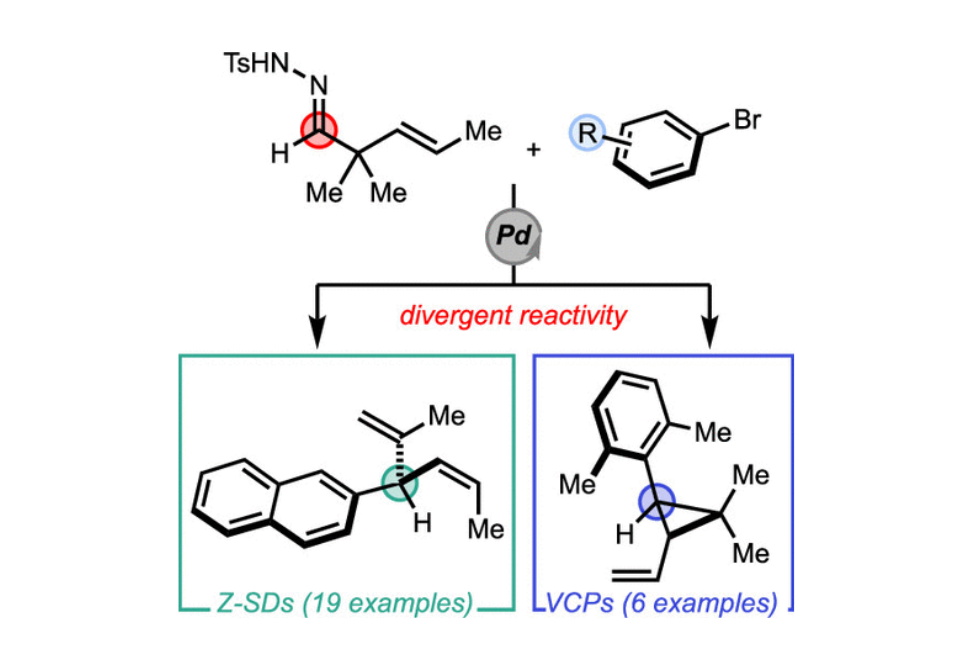
Divergent Reactivity of α,α-Disubstituted Alkenyl Hydrazones: Bench Stable Cyclopropylcarbinyl Equivalents
Abstract
Herein we report the divergent reactivity of 2,2-dialkyl-3-(E)-alkenyl N-tosylhydrazones using Pd-catalyzed cross-coupling conditions, which enable the Z-selective synthesis of 3-aryl-1,4-dienes and gem-dialkyl vinylcyclopropanes. We found that the dialkylbiaryl phosphine ligand SPhos was the optimal ligand for this transformation producing skipped dienes in up to 83% isolated yield. The ratio of skipped diene to vinylcyclopropane is dependent on both the structure of the α,α-disubstituted hydrazones and the aryl halide partner. Using sterically encumbered aryl bromides provided the trans-cyclopropane products selectively in up to 69% yield. The reaction is stereospecific and stereoselective and occurs alongside a competing 1,2-alkenyl group migration pathway.
Citation
Divergent Reactivity of α,α-Disubstituted Alkenyl Hydrazones: Bench Stable Cyclopropylcarbinyl Equivalents
Nina F. C. Ritchie, Adam J. Zahara, and Sidney M. Wilkerson-Hill
Journal of the American Chemical Society Article ASAP
DOI: 10.1021/jacs.1c12881