Direct C-H Radiocyanation of Arenes via Organic Photoredox Catalysis
Abstract
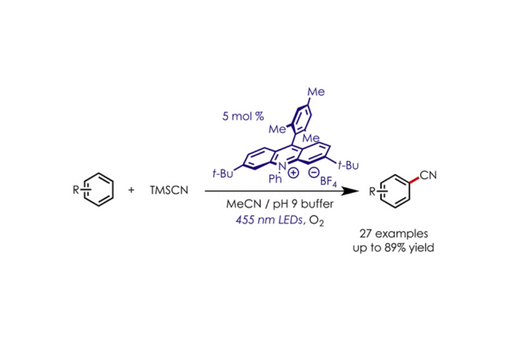
Methods for the direct C–H functionalization of aromatic compounds are in demand for a variety of applications, including the synthesis of agrochemicals, pharmaceuticals, and materials. Herein, we disclose the construction of aromatic nitriles via direct C–H functionalization using an acridinium photoredox catalyst and trimethylsilyl cyanide under an aerobic atmosphere. The reaction proceeds at room temperature under mild conditions and has proven to be compatible with a variety of electron-donating and -withdrawing groups, halogens, and nitrogen- and oxygen-containing heterocycles, as well as aromatic-containing pharmaceutical agents.
Citation
Direct C–H Cyanation of Arenes via Organic Photoredox Catalysis
Joshua B. McManus and David A. Nicewicz
Journal of the American Chemical Society 2017 139 (8), 2880-2883
DOI: 10.1021/jacs.6b12708