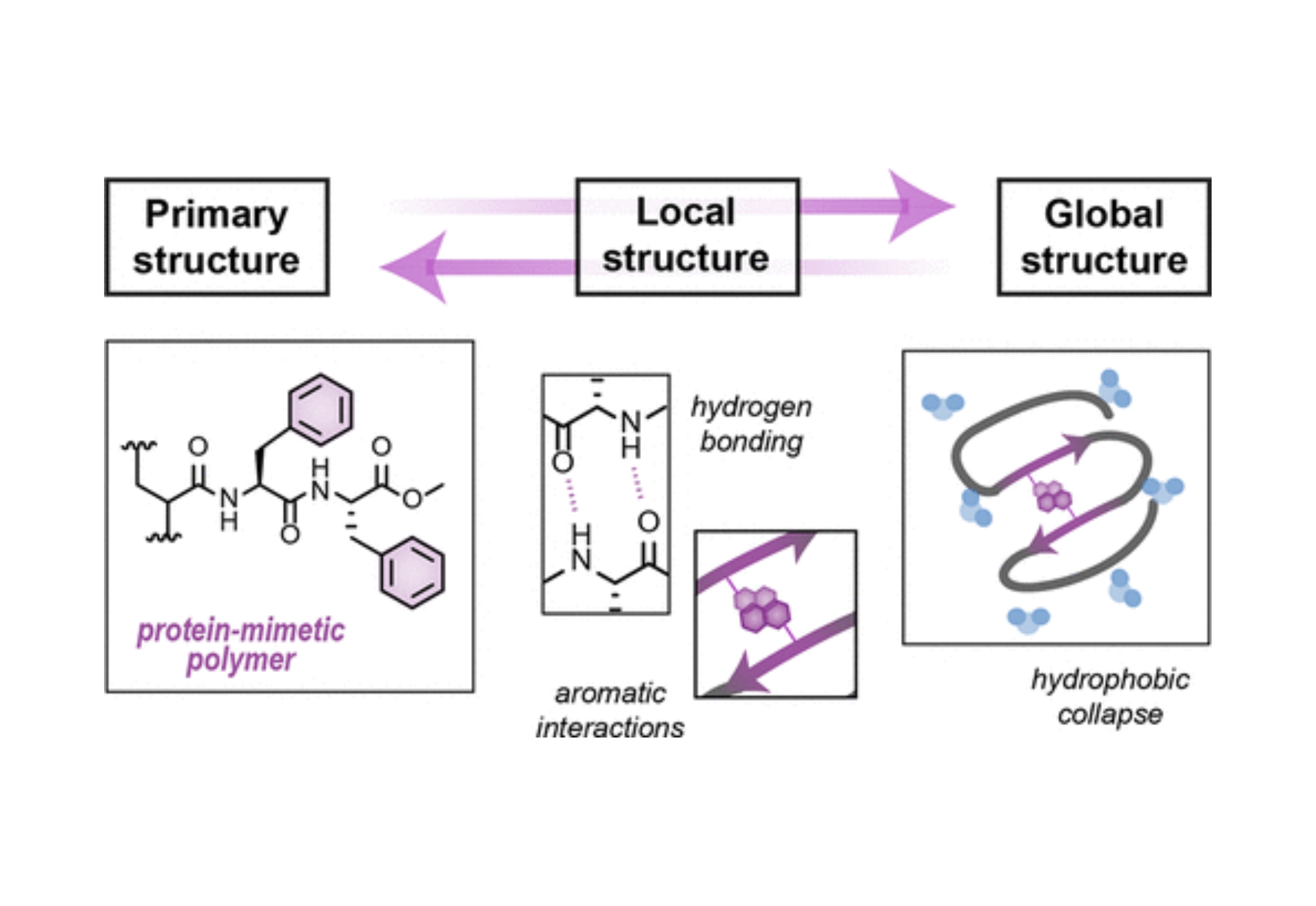
Controlling Amphiphilic Polymer Folding beyond the Primary Structure with Protein-Mimetic Di(Phenylalanine)
Congratulations to postbaccalaureate researcher Jacqueline Warren and graduate student Peter Dykeman-Bermingham on their recent publication describing the development of amphiphilic copolymers with protein-mimetic higher-order structure! Despite advances increasing the monomer scope available to synthetic polymers, our ability to control higher order structure analogous to those that dictate protein function remains limited. The dipeptide di(phenylalanine) is a key component of fibrilization in a protein known to misfold in Alzheimer’s disease and is known to alone induce β-sheet interactions. Inspired by this, di(phenylalanine) was introduced as a sidechain to drive β-sheet-like interactions within synthetic amphiphilic polymers. Characterization of these copolymers alongside a series of control polymers with related hydrophobic moieties revealed that hydrophobicity was the predominant driving force for the collapse of amphiphilic polymers. Multiple characterization techniques revealed polymers containing the di(phenylalanine) moiety had the most local (secondary-like) structure. The team is excited to continue answering questions about how this unique monomer can control the internal structure of amphiphilic polymers, and what role that can play in polymer function.
Citation
Controlling Amphiphilic Polymer Folding beyond the Primary Structure with Protein-Mimetic Di(Phenylalanine)
Jacqueline L. Warren, Peter A. Dykeman-Bermingham, and Abigail S. Knight
Journal of the American Chemical Society 2021 143 (33), 13228-13234
DOI: 10.1021/jacs.1c05659