Genevieve Holliday Reveals How Small Material Changes Can Greatly Improve Solar Efficiency
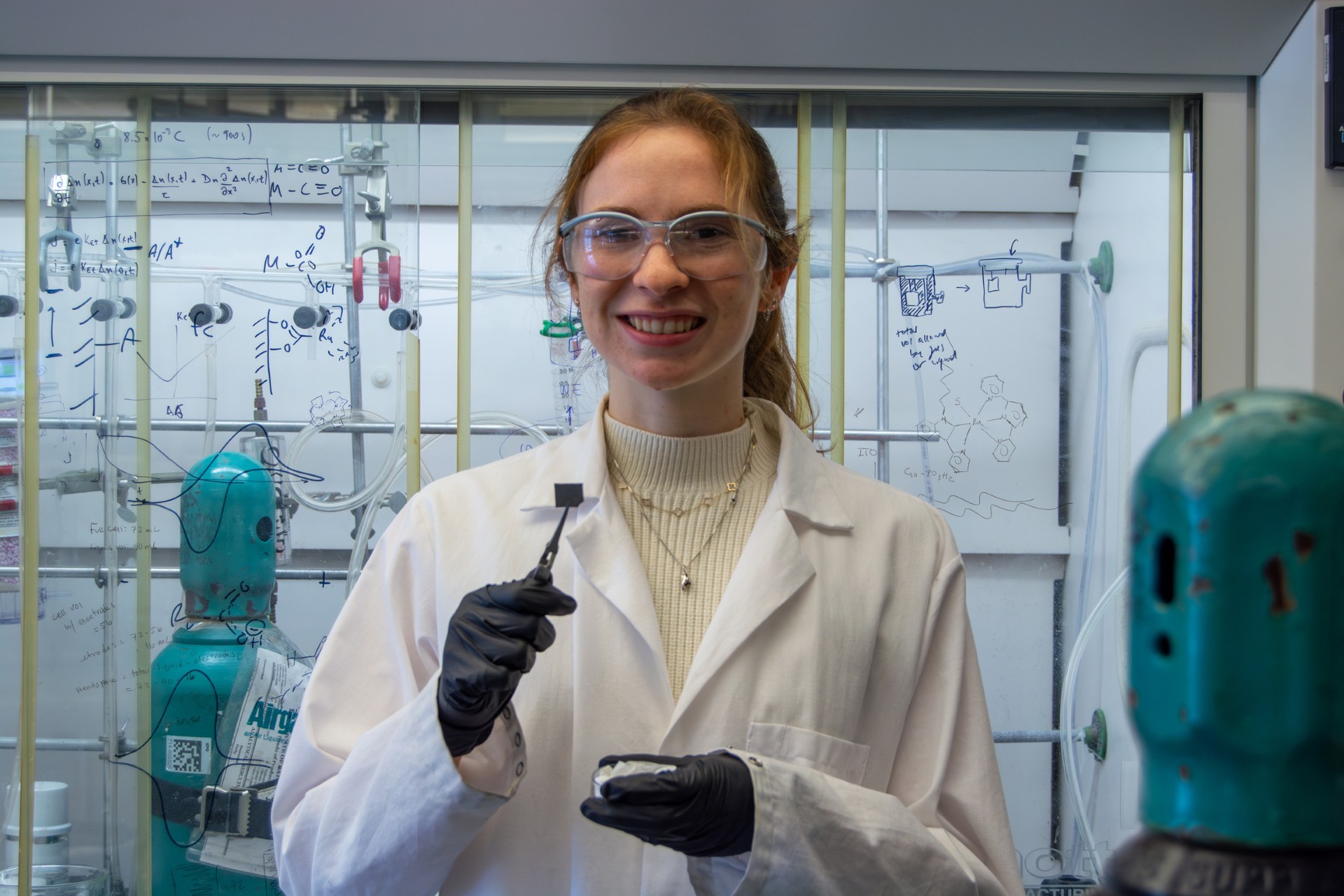
In the Dempsey Lab, Genevieve Holliday, a chemistry major at the University of North Carolina, focuses on the tiny interface where electrodes, which are the conductive surfaces that move electric charge, meet catalysts, which are the molecules that facilitate lower-energy barrier chemical reactions. Her goal is to understand how electrons move between these surfaces—a process called interfacial electron transfer—and how that affects fuel production.
November 4, 2025 I By Dave DeFusco
When Genevieve Holliday arrived at the University of North Carolina, she wasn’t sure what kind of scientist she wanted to be. She knew she loved chemistry and physics, but she had never set foot in a research lab. That changed her second semester when she joined the Erie Lab, where she used a technique called capillary electrophoresis to study how proteins and DNA interact.
“It was my first experience in research,” said Holliday, who is majoring in chemistry. “Biochemistry was interesting, but I realized I didn’t want to go into genetics. I found myself drawn more to my physics classes, and I started thinking about energy and materials science.”
That curiosity led her to the Dempsey Lab, part of the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), a national collaboration of scientists working to use sunlight to convert carbon dioxide and water into liquid fuels. It’s an ambitious mission: to design systems that can store solar energy in chemical form, creating sustainable fuels that could one day replace gasoline.

For Holliday, the attraction was immediate. “I grew up excited about clean energy,” she said. “I’d thought about solar panels and wind turbines, but I didn’t realize sunlight could be stored directly as a fuel. That idea—that we could replace gasoline with something made from sunlight—was amazing to me.”
In the Dempsey Lab, Holliday focuses on the tiny interface where electrodes, which are the conductive surfaces that move electric charge, meet catalysts, which are the molecules that facilitate lower-energy barrier chemical reactions. Her goal is to understand how electrons move between these surfaces—a process called interfacial electron transfer—and how that affects fuel production.
“My first semester was a crash course in cyclic voltammetry,” she said, referring to a method that measures how easily electrons transfer to molecules in solution. “We were testing coatings of amorphous titanium dioxide on silicon electrodes to see how thick they could be before they started blocking charge transfer. A thicker coating meant more stability, but at the cost of blocking charge transfer.”
Now, she uses time-resolved infrared spectroscopy, a laser-based technique that captures what happens at the surface of a material in millionths of a second. “It’s elegant,” she said. “You’re probing a surface with light and trying to read what it tells you. You can watch how charge carriers behave in real time.”
Jillian Dempsey, Bowman and Gordon Gray Distinguished Term Professor, said Holliday is the first researcher in her lab to learn time-resolved infrared spectroscopy. “Her work is helping us understand how seemingly small differences in the way we interface catalysts with silicon light absorbers can have a big impact on charge carrier lifetimes,” said Professor Dempsey. “Her findings are helping us rationalize why some hybrid photoelectrodes operate more efficiently than others.”

Holliday’s research explores how small changes in surface structure, like adding carbon chains of different lengths, can affect how long charge carriers stay active at the surface. “The longer those electrons live, the more useful work they can do,” she said. “We’re trying to figure out what makes some surfaces better at maximizing those electron lifetimes.”
Last summer, Holliday interned at the National Institute of Standards and Technology (NIST) in Boulder, Colo., as part of a summer undergraduate research fellowship. There, she studied 3D imaging methods for semiconductor chips using Focused Ion Beam-Secondary Ion Mass Spectrometry (FIB-SIMS), a high-precision tool for examining materials at the nanoscale.
“It was very different work from what I do at UNC,” she said. “But learning a new technique from scratch helped me come back to my own lab with a fresh perspective. It reminded me how stepping outside your field can make you see your own work differently.”
Across all her experiences—in biology, chemistry, physics and materials science—Holliday sees a common thread: collaboration. “Physicists and chemists talk about the same problems in different languages,” she said. “I’ve learned to ask a lot of questions, even if I might think I know the answer. That’s how science moves forward.”
As a Morehead-Cain Scholar, Holliday is grateful for the opportunities that have shaped her as both a thinker and a collaborator. Whether she goes to graduate school or joins the clean energy industry, she knows her future will be interdisciplinary.
“All these experiences—working in labs, learning new techniques, collaborating across fields—feel connected now,” she said. “I don’t know exactly where I’ll go next, but I know I want to be part of solving the clean energy challenge. That’s what keeps me excited every day in the lab.”