Mismatched covalent and noncovalent templating leads to large coiled coil-templated macrocycles
Abstract
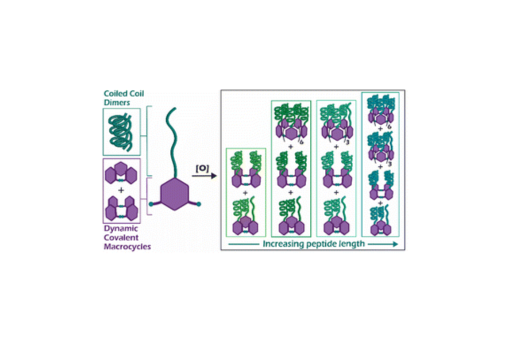
Herein we describe the use of dynamic combinatorial chemistry to self-assemble complex coiled coil motifs. We amide-coupled a series of peptides designed to form homodimeric coiled coils with 3,5-dithiobenzoic acid (B) at the N-terminus and then allowed each B-peptide to undergo disulfide exchange. In the absence of peptide, monomer B forms cyclic trimers and tetramers, and thus we expected that addition of the peptide to monomer B would shift the equilibrium towards the tetramer to maximize coiled coil formation. Unexpectedly, we found that internal templation of the B-peptide through coiled coil formation shifts the equilibrium towards larger macrocycles up to 13 B-peptide subunits, with a preference for 4, 7, and 10-membered macrocycles. These macrocyclic assemblies display greater helicity and thermal stability relative to intermolecular coiled coil homodimer controls. The preference for large macrocycles is driven by the strength of the coiled coil, as increasing the coiled coil affinity increases the fraction of larger macrocycles. This system represents a new approach towards the development of complex peptide and protein assemblies.
Citation
Stingley, K. J., Carpenter, B. A., Kean, K. M., & Waters, M. L. (2023). Mismatched covalent and noncovalent templating leads to large coiled coil-templated macrocycles. Chemical Science, 14(18), 4935–4944. https://doi.org/10.1039/d3sc00231d