Department News

Nicholas Boyer, a rising senior at UNC-Chapel Hill double majoring in chemistry and computer science was selected to receive a 2024 Barry Goldwater Scholarship.

Photos of Chemistry student and faculty volunteers at UNC's 75th annual Science Expo.
Research

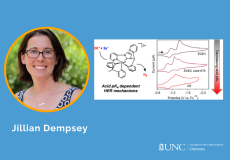
In the invited Perspective article for the Journal of the American Chemical Society, members of the Kanai research group discuss...
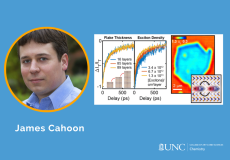
Transition metal dichalcogenides (TMDCs) have garnered considerable interest over the past decade as a class of semiconducting layered materials.
Our Faculty
Faculty in the Department of Chemistry at the University of North Carolina help define solutions to the pressing scientific problems of the day. A significant and key component of our department’s strategic plan is to cultivate the next generation of scientific leadership. Faculty, from our assistant professors to our most senior and distinguished colleagues, are international leaders in their subfields, garnering local, national, and international recognition and accolades commensurate with their excellence in research and teaching.
Our Graduate Students
Our graduate students form the next generation of scientific leaders. As a department, we seek to recruit and mentor a diverse cohort of students dedicated to excellence in the classroom and research laboratory. The creativity, drive, collegiality, and accomplishments of our graduate students in tackling difficult scientific problems are significant reasons why UNC is an international leader in chemical research.