Faculty Members

Jeffrey Johnson
A. Ronald Gallant Distinguished Professor
Caudill Laboratories 220919-843-4936
jsj25@email.unc.edu
Group Website
Curriculum Vitae
Research Interests
Synthetic Organic Chemistry, Asymmetric Catalysis
Research Synopsis
My interests lie in the general area of chemical synthesis. I am particularly interested in the discovery of new organic transformations and their application to the total synthesis of architecturally challenging and biologically important natural products. Our criteria for the development of a synthetic method may be summarized by three characteristics:
One
The reaction should achieve several structural modifications in a single synthetic operation: tandem or domino reactions are optimal;
Two
The reaction should introduce a significant level of stereochemical complexity in a controlled fashion and absolute stereocontrol should arise from a chiral source used in catalytic quantities;
Three
The starting materials should be readily accessed, but be easily transformed into complex products. To meet these challenges, students in my group will learn a full complement of organic and organometallic chemistry.
Professional Background
B.S., University of Kansas, 1994; Ph.D., Harvard University, 1999; NIH Postdoctoral Fellow, University of California, Berkeley, 1999-2001; Research Corporation Research Innovation Award, 2002; National Science Foundation CAREER Award, 2003-2008; 3M Nontenured Faculty Award, 2003; UNC Junior Faculty Development Award, 2004; Johnson and Johnson Focused Giving Award, 2004-2006; Eli Lilly Grantee, 2004; Amgen Young Investigator Award, 2005; GSK Scholar Award, 2006-2007; Alfred P. Sloan Fellow, 2006-2008; Camille Dreyfus Teacher Scholar, 2006-2009; Ruth and Phillip Hettleman Prize for Artistic and Scholarly Achievement, 2006; Novartis Early Career Award in Organic Chemistry, 2008; American Chemical Society's Arthur C. Cope Scholar Award in Recognition of Excellence in Organic Chemistry, 2010; Elias J. Corey Award, 2012; Society of Synthetic Organic Chemistry, Japan Lectureship Award, 2014; Japanese Society for the Promotion of Science Fellow, 2015; William C. Friday, Class of 1986, Award for Excellence in Teaching, 2016, Journal of Organic Chemistry Outstanding Author of the Year 2016, AAAS Fellow 2019
Research Group
News & Publications
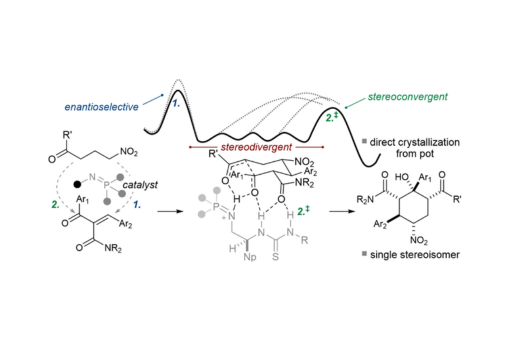
A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.
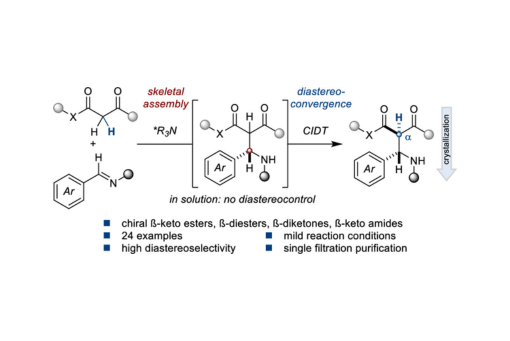
Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Brian Hogan
Teaching Professor, Vice Chair of Education
Kenan Laboratories C348919-962-4746
hoganb@email.unc.edu
Curriculum Vitae
Research Interests
Biochemistry Education & Undergraduate Research Skills Development
Research Synopsis
Dr. Hogan has thrived while teaching at Carolina, winning three Tanner Teaching Awards for Excellence in Undergraduate Education, the Chemistry Department teaching award, the University Diversity Award, the Robert E. Bryan Award for Extraordinary Public Service and Engagement, and the Chapman Family Faculty Fellowship for teaching excellence. He is also a faculty fellow in the UNC Institute of Arts and Humanities as well as the Carolina Center for Public Service.
His research focus on the effects of positive role modeling on Middle and High School students through a series of STEM related outreach programs. Brian engages students in the disciplines of biochemistry and molecular biology by teaching a wide variety of courses, spanning multiple disciplines, and helps prepare students for the future through his role as the director of several mentoring programs.
Professional Background
Trenton State College, B.S., 1996; Research Assistant, Waksman Institute of Microbiology, Rutgers University; Research Assistant. Iconix Pharmaceuticals; University of North Carolina, Ph.D., 1999-2003; Visiting Lecturer, Department of Chemistry, University of North Carolina, 2003-2004; Tanner Award for Excellence in Undergraduate Teaching, 2017; Tanner Award for Excellence in Undergraduate Teaching, 2010; Chapman Family Faculty Fellowship, 2010
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Leslie Hicks
Sherman Fairchild Foundation Chancellor's Scholar Term Associate Professor
Caudill Laboratories 339919-843-6903
lmhicks@unc.edu
Group Website
Curriculum Vitae
Research Interests
Proteomics, Posttranslational Modifications, Mass Spectrometry
Research Synopsis
The ability to exquisitely differentiate biological molecules dictating metabolism and its underlying biochemistry is a challenging and meaningful endeavor, as it underpins both fundamental biological research and applied bioengineering. With an interest in extending biological frontiers using advanced technologies, the Hicks lab aims to establish methods, methodologies, and concepts to set the foundation for clever, practical, and meaningful applications of mass spectrometry in addressing and answering important biological questions.
Professional Background
Dr. Hicks received her B.S. in Chemistry at Marshall University, summa cum laude, and Ph.D. in Analytical Chemistry at the University of Illinois, Urbana-Champaign where she was the recipient of an NSF Graduate Research Fellowship. She was an Assistant Member and Principal Investigator at the Donald Danforth Plant Science Center and an adjunct professor in the Department of Biology at Washington University in St. Louis prior to assuming her current role as a professor in the Department of Chemistry at UNC.
Research Group
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Gary Glish
Professor
Caudill Laboratories 320919-962-2303
glish@unc.edu
Group Website
Curriculum Vitae
Research Interests
Mass Spectrometry, Ion Chemistry, Biomolecule Analysis
Research Synopsis
Our research focuses on the development of instrumentation and methods in the field of mass spectrometry. There is a strong synergism between the research involving development of new instrumentation, or modification of existing instrumentation, and the applications. Development of new instrumentation allows us to do novel experiments in the characterization of different chemical systems, while understanding the needs for analysis of these systems drives the development of the next generation of instrumentation and methods. Currently the primary focus in instrument/methods development involves ion mobility, ambient ionization sources, and ion activation.
In instrument development we are especially interested in the fundamentals and applications of differential ion mobility spectrometry, DIMS. DIMS, combined with mass spectrometry, is a powerful tool for targeted trace analyte detection and real-time analysis of complex samples. In the area of ambient ionization, we have been working primarily with techniques we can use for real-time characterization of aerosols. The techniques include extractive electrospray ionization, low temperature plasma ionization, and a new method we are developing, liquid overflow capture electrospray ionization.
We are applying both DIMS and ambient ionization to the characterization of aerosols, from both the biological and environmental perspective. We have interests in characterizing the burning of biomass, for example tobacco and emerging tobacco products. Within that realm we have a major focus on characterizing e-cigarette aerosols. In more biological applications we are involved in using DIMS/MS for the discovery and detection of leukemia antigen peptides with a goal toward application to personalized medicine, i.e. tailoring the patient treatment based on actual antigens being presented by the patient. Characterization of lipids via DIMS/MS is another biological area of interest.
In everything we do we are interested in the underlying chemistry involved, and the mass spectrometer is a rich source of unique chemistry. From the aerosol project a small unique peak in an MS/MS spectrum has led to a whole new project on the differentiation of stereoisomers by a simple ion/molecule reaction: no separations, no special chiral reagents or transition metals, just water. As part of the fundamental chemistry studies we complement a lot of our experimental work with theoretical calculations, DFT, collision cross-section, ion trajectories, et cetera.
Professional Background
Ph.D, Purdue University, 1980; Research Scientist and Group Leader, Oak Ridge National Laboratory, 1980-1992; President, American Society for Mass Spectrometry, 2008-2010; Vice President for Programs, American Society for Mass Spectrometry, 2006-2008; Associate Editor, Journal of the American Society for Mass Spectrometry, 1990-2007; Chair, International Workshop on MS/MS, 1999-2007; Vice President for Arrangements, American Society for Mass Spectrometry, 1987-89; Board of Directors, Asilomar Conf. on Mass Spectrometry, 1987-1989; Editorial Advisory Board, Instrumentation Science and Technology, 1993 - present.
Research Group
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Michel Gagné
Mary Ann Smith Distinguished Professor of Chemistry
Caudill Laboratories 218919-962-6341
mgagne@unc.edu
Group Website
Curriculum Vitae
Research Interests
Catalysis, Synthetic Methods, Synthetic Receptors, Biofuels
Research Synopsis
Research in the Gagné group focuses on catalysis and synthetic methods. We develop catalytic methods for carrying out selective transformations in complex environments under catalyst, such as kinetic, control. We seek to control where a catalyst reacts and what reaction/s it promotes in multi-functional environments to modify complex structures for many applications; we are currently concentrating on biomass conversion and the late-stage functionalization of bioactive molecules. We also have interests in de novo design of peptide-based catalysts to create artificial enzymes, and new organometallic silyl complexes.
Biomass Conversion
Here we seek to convert biorenewable carbon sources such as cellulose into high-value chemicals, which are currently accessed from petroleum roots. Since the most valuable chemicals are partially oxidized, they are accessed by the oxidation of petroleum resources. On the other hand, biomass is an over-oxidized source of carbon, each carbon in glucose is at the alcohol or aldehyde oxidation state, and so the challenge is to develop chemistries for reducing, or more precisely, deoxygenating biomass.
To achieve this goal in a site-selective manner, we have turned to non-transition metal catalysts for the activation of silanes. The fluoroaryl borane catalyst B(C6F5)3 is unique in its ability to heterolytically activate silanes, and we have used it to develop strategies for the site-selective deoxygenation of cellulosic biomass. We have also discovered that hydro-boranes can be activated in the same way as hydro-silanes for deoxygenation chemistry. The following Venn diagram shows the compounds we have directly synthesized from a primary biorenewable, glucose, sorbitol, isosorbide, et cetera. Silanes and boranes exhibit unique selectivities.
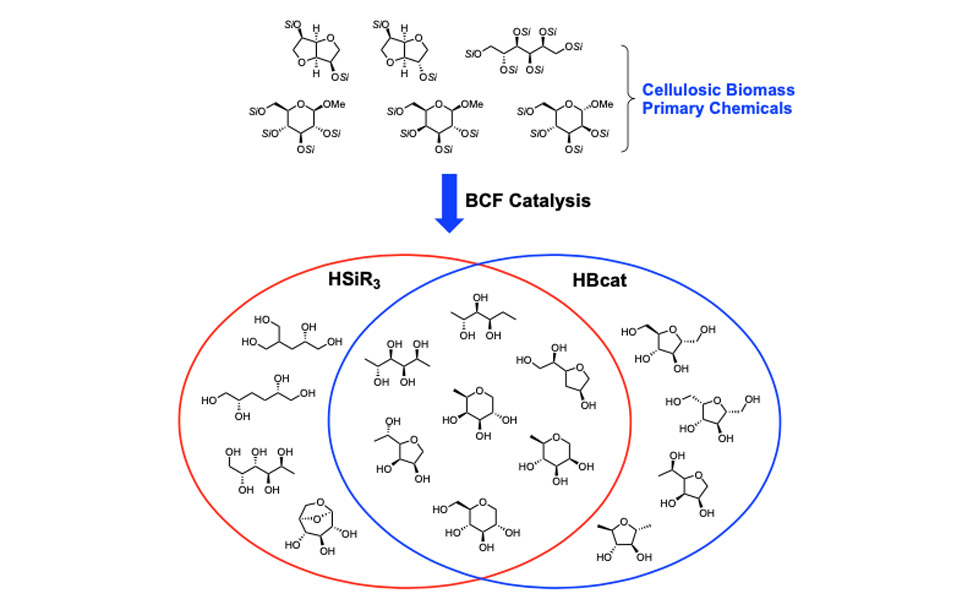
Late-Stage Functionalization
The strategies we have developed for achieving site-selectivity in poly-ols, also serves us well in our goals of site- and chemo-selective reactivity in poly-functional bioactive natural products. Here the challenges are magnified as there are many more potentially reactive functional groups. We seek catalyst control over site-selectivity in complex natural products as it enables late stage functionalization, which provides structurally modified compounds for structure activity relationship, SAR, studies, optimization of biological efficacy, bioavailability, et cetera. In the example described below the anti-fungal natamycin is modified at different sites depending on which catalyst is used.
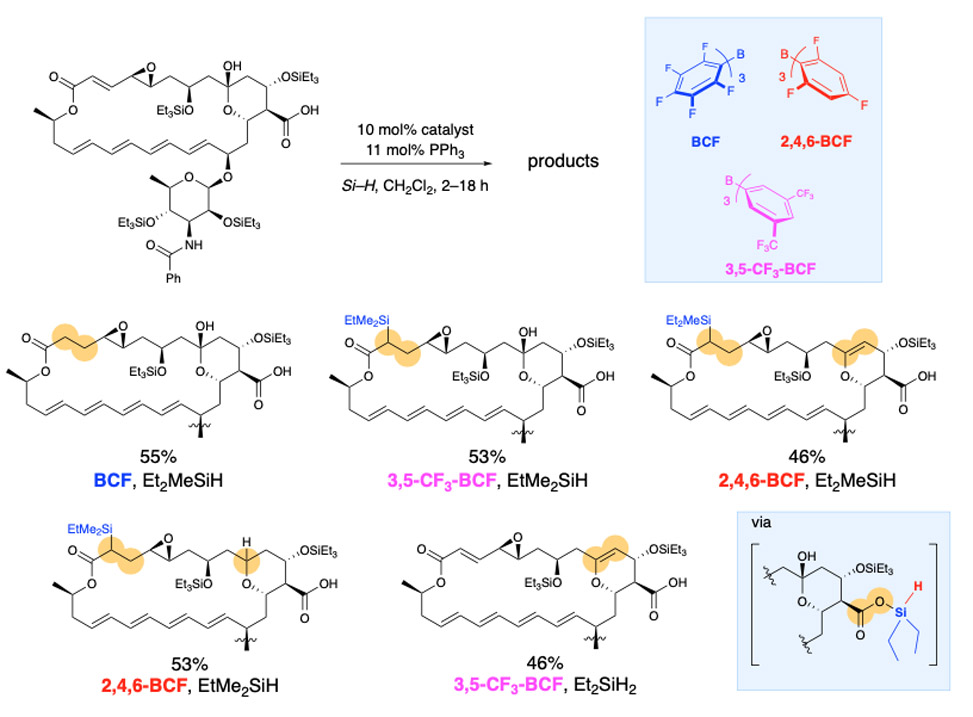
Professional Background
Ph.D., Northwestern University, 1991; NSERC of Canada Postdoc at Caltech, 1991-1992, and Harvard University, 1992-1995; CaRLa Fellow, University of Heidelberg, 2008; Exeter College Visiting Fellow, Oxford University, 2006; SCR Member Wadham College, Oxford University, 2005-2006; W. R. Kenan Jr. Leave, 2005-2006; NSERC GSC-24 Grants Selection Committee, 2005-2008; Canadian Journal of Chemistry Board of Editors, 2002-2005; Camille-Dreyfus Teacher Scholar Award, 2000; Union Carbide Innovation Recognition Award, 2000; 3M Untenured Faculty Award, 1999; NSF Career Award, 1996; Fellow, American Association for the Advancement of Science, 2012; Royal Society of Chemistry, “Catalysis in Organic synthesis” Award, 2016; Fellow, Royal Society of Chemistry, 2016.
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Dorothy Erie
Professor
Genome Sciences Building 4360919-962-6370
derie@email.unc.edu
Group Website
Curriculum Vitae
Research Interests
Scanning Force Microscopy, Biological Chemistry
Research Synopsis
The research in my lab is divided into two main areas:
- Atomic force microscopy and fluorescence studies of protein-protein and protein-nucleic acid interactions.
- Mechanistic studies of transcription elongation. My research spans the biochemical, biophysical, and analytical regimes.
Listed below is an outline of research topics. More detailed information about each topic is included on my group page.
Atomic Force Microscopy
Studies of the structure-function relationship of protein-protein and protein-DNA interactions related to DNA repair
Fluorescence Spectroscopy
Development of a combined AFM-fluorescence microscope for the study of multi-protein systems
Single-molecule fluorescence spectroscopy of protein-DNA complexes
Transcription Elongation
Transient-state kinetic studies of single and multiple nucleotide incorporation
Characterization of RNA polymerases from thermophilic bacteria
Professional Background
B.S. Louisiana State University, 1982; M.S. University of Wisconsin, 1985; Ph.D., Rutgers, 1989
Research Group
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Jillian Dempsey
Bowman and Gordon Gray Distinguished Term Professor, Director of Undergraduate Studies
Kenan Laboratories 440919-962-4617
dempseyj@email.unc.edu
Group Website
Curriculum Vitae
Research Interests
Inorganic Spectroscopy and Solar Energy Conversion
Research Synopsis
Research in the Dempsey group applies the tools of physical inorganic chemistry to address challenges associated with developing efficient solar energy conversion processes. In particular, we focus on understanding the proton-coupled electron transfer reactions that underpin fuel production and elucidating electron transfer processes across materials interfaces. Our research program bridges molecular and materials chemistry and relies heavily on methods of physical inorganic chemistry, including electrochemistry and time-resolved optical spectroscopy.
Professional Background
Massachusetts Institute of Technology, BS, 2005; California Institute of Technology, PhD, 2011; NSF American Competitiveness in Chemistry Postdoctoral Fellow at the University of Washington, Seattle, 2011-2012; UNC Junior Faculty Development Award, 2015, NSF CAREER Award, 2015; Packard Fellowship for Science and Engineering, 2015; Air Force Office of Scientific Research Young Investigator Award, 2016; Sloan Research Fellowship, 2016
Research Group
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

James Cahoon
Hyde Family Foundation Professor
Caudill Laboratories 19919-962-3386
jfcahoon@unc.edu
Group Website
Curriculum Vitae
Research Interests
Nanomaterials/Nanowire Synthesis, Solar & Thermal Energy, Photonics
Research Synopsis
My research program is focused on the chemical synthesis of semiconductor nanomaterials with unique physical properties that can enable a range of technologies, from solar cells to solid-state memory. My Ph.D. background is in experimental physical chemistry, and my post-doctoral training focused on nanomaterial synthesis, a topic at the border of physical chemistry, inorganic materials, and engineering.
At UNC, I have combined these backgrounds to develop a research program that emphasizes nanomaterials synthesis coupled with detailed physical characterization and computational modeling. By combining these three key areas - synthesis, measurement, and modeling - we have a general strategy to develop new materials with specific properties and function under the overarching theme "morphology encodes function."
Because the materials we develop have dimensions on the nanometer length scale, subtle changes in size, geometry, and composition have profound influence on the optical and electrical characteristics of the materials; thus, we use morphology as a tool to rationally encode specific physical properties, which we predict and model through computation.
The research areas and directions currently pursued include:
1. Synthesis
We develop new methods and tools for the synthesis of semiconductor nanomaterials. For example, we constructed a new class of chemical vapor deposition system that uses heat, light, or electric plasmas to drive nanomaterial formation from organic and inorganic vapor-phase and solid-state precursors. We study the fundamental mechanisms of nanomaterial growth processes, for instance, by combining kinetic measurements with models that identify the rate-limiting steps during nanomaterial formation, enabling rational control of the growth process.
2. Measurement
We perform optical and electrical measurements on single-nanostructures as well as on ensemble systems. By measuring physical properties from the nanoscale to mesoscale to macroscale, we can understand the progressive evolution of physical characteristics. For instance, optical absorption and light scattering characteristics from isolated nano-objects progressively change when placed in 1-dimensional arrays or arranged into 2-dimensional periodic structures.
3. Modeling
Finite-element and ab-initio computational modeling is used to create realistic, three-dimensional representations of our semiconductor nanomaterials. We apply physics, as needed, in our models to both interpret physical measurements and to predict new properties that guide the synthesis of materials. Two key areas in which we apply our models are optics, in which we can use full electromagnetic wave simulations to predict absorption, scattering, and waveguiding properties, and electronics, in which we use simulations to predict the flow of current, charge carrier diffusion and recombination processes, etc, within single nanostructures.
4. Photonics
We design and characterize semiconductor structures that fulfill specific photonic or plasmonic functions. For instance, we chemically synthesize structures that can be used for surface-enhanced Raman spectroscopy or as photonic-crystal cavities. We also examine the plasmonic characteristics of degenerately-doped semiconductors, such as phosphorus-doped silicon nanowires, which can serve as a new class of materials to control light propagation at sub-diffraction-limited length scales.
5. Electronics
Using our ability to dope semiconductor nanostructures and chemically encode morphology, we are developing electronic devices with specific functionality. For example, we have created nanowires with constrictions that behave as non-volatile memory and exhibit a resistive-switching effect. In addition, we have created nanowire photodiode detectors with wavelength-tunable response by encoding p-n junctions in nanowires with specific diameters and morphologies that exhibit high-Q optical resonances in the visible to near-infrared wavelength ranges.
6. Photovoltaics
We are developing advanced photovoltaic devices based on nanostructured, quantum-confined group IV materials. Through a combination of thermal and plasma-enhanced chemical vapor deposition, we can synthesize nanowires and quantum-dots within the same reactor to create complex, hybrid materials with characteristics length scales from a few nanometers to tens of microns - all within the same structure. These efforts lay the groundwork for a new class of generation III photovoltaic devices.
7. Solar Fuels
Our efforts are integrated with the UNC Energy Frontier Research Center for Solar Fuels to develop p-type metal-oxide nanomaterials with morphologies and compositions designed for integration with molecular dyes and catalysts in solar fuel devices. For instance, using a nanoplatelet morphology, we have improved charge carrier mobilities by one order of magnitude, and using core/shell structures, for example NiO/Al2O3; NiO/Cu2O, we have improved charge carrier lifetimes by three orders of magnitude.
8. Dynamics
Through a joint effort with Prof. John Papanikolas at UNC-Chemistry, we are using ultrafast microscopy to probe fundamental charge carrier dynamics in single nanostructures on the femtosecond, picosecond, and nanosecond time scales. By combining the microscopy with rationally-designed materials and computational modeling of the dynamics, we have a comprehensive strategy to understand the dependence charge carrier flow and recombination on parameters such as composition, size, strain, etc, in single nano-objects.
Professional Background
B.S. Chemistry & Philosophy, College of William and Mary, 2003; Ph.D. Physical Chemistry, University of California-Berkeley, 2008; IC Postdoctoral Fellow, Harvard University, 2009-2011; Barry M. Goldwater Scholarship, 2002; National Science Foundation Graduate Research Fellowship, 2004; ACS Physical Chemistry Division Post-doctoral Research Award, 2010; Packard Fellowship for Science and Engineering, 2014; Sloan Research Fellowship, 2015; Cottrell Scholar Award, 2015; NSF CAREER Award, 2016
Research Group
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Carribeth Bliem
Teaching Associate Professor
Kenan Laboratories 147B919-962-6194
bliem@email.unc.edu
Curriculum Vitae
Research Interests
Chemistry Education, Curriculum Development
Research Synopsis
My current teaching efforts are centered on bringing evidence-based practice to the undergraduate classroom, especially in large-enrollment courses. To that end, I’ve been involved in curriculum reform of our first-year general chemistry courses, implementing a high-structure active-learning environment designed to help students learn to think critically about problems and work collaboratively to find solutions.
These same themes extend into my upper-level physical chemistry courses. My goals are that students gain what they need in order to achieve their career goals and that they see the relevance of chemistry to daily life.
Professional Background
B.S. Chemistry, University of Utah, 1985.; M.S. Chemistry, University of Colorado at Boulder,1989. Visiting Lecturer, University of North Carolina at Chapel Hill, 2002-2017. Teaching Assistant Professor, 2018-present.
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.

Joshua Beaver
Teaching Associate Professor
Kenan Laboratories 147F919-962-3663
jebeaver@live.unc.edu
Curriculum Vitae
Research Interests
Chemistry Education, Organic Chemistry, Curriculum Development, Chemistry Education Research
Research Synopsis
Myriad fundamental chemical interactions cooperate and compete to drive biological processes. My chemistry research interests combine organic and biological chemistries to probe the intermolecular interactions between large molecules in water.
My graduate work focused on building our understanding of the contribution of aromatic, hydrophobic, and hydrogen-bonding interactions to protein-substrate binding and my postdoctoral training translated this work to the development of targeted anticancer drug delivery agents using DNA-based nanoparticles.
As a teaching professor I adapt this interdisciplinary problem-solving approach to active, evidence-based learning within the classroom. My highest priority as an educator is to provide my students with opportunities to take an active role in learning. My goal is to help students understand chemistry by developing translatable problem solving and critical thinking skills that will benefit them both in and outside of the classroom.
As a teaching professor at UNC, I am involved in several clinical research and curriculum development projects. One project is in collaboration with researchers in the School of Education and the Office of Digital and Lifelong learning to create data-driven analytics that correlate student success with their resource use, early performance on low-stakes assessments, and overall course engagement early in the semester. We then use these models to identify students that benefit from additional support or interventions at early stages of the semester to improve their trajectory and ultimately their learning and performance in the course. Other projects include deepening our understanding of modes of instruction and how they influence student learning, developing and establishing effective teaching methodologies regardless of instructor, and developing novel scaffolded learning resources for CHEM 261/262.
Professional Background
B.S. Chemistry, Juniata College, 2009; Ph.D., Organic Chemistry, NSF Graduate Research Fellow, University of North Carolina at Chapel Hill, 2014; Postdoctoral Researcher, Duke University, 2014-2016; Teaching Assistant Professor, University of North Carolina at Chapel Hill, 2016-present, Student Undergraduate Teaching and Staff Award for excellence in teaching, 2023, and Tanner Award for Excellence in Undergraduate Teaching, 2024.
News & Publications

A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.

Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.